In 1991, a research group lead by MIT professor James Fujimoto came up with the idea of the OCT principle, and first demonstrated OCT in living human eyes in 1992. According to Eric Swanson, one of the co-inventors: ‘Today, approximately 30 million OCT imaging procedures are performed worldwide each year, and the OCT market is approaching $1 billion a year.’
Research has shown that OCT has saved billions of dollars of healthcare expenditures and has led to improved understanding and treatments of various diseases in areas such as ophthalmology, cardiology, surgical guidance and cancer.
With OCT, low coherence light is radiated into tissue and the backscattered light is evaluated using an interferometer. The contrast in the tissue comes from scattering processes at structural boundaries, where the refractive indices change. A wavelength range in the near infrared at 800 to 1,350nm is typically used in order to penetrate tissue safely.
In an OCT interferometer, the light beam is split. One partial beam acts as a reference arm, the other is directed to the sample, reflected from there and superimposed on the first. Two variants are available for the interferometric evaluation of this measurement: Time Domain OCT (TD-OCT) and frequency domain OCT (FD-OCT) and the latter divided into the subareas spectral domain OCT (SD-OCT) and swept source domain OCT (SS-OCT).
With TD-OCT, the length of the reference arm, and thus the propogation time of the reference light, is changed. If the runtime of the reference beam and of the beam reflected by the sample match, then they interfere constructively. The broader the wavelength range of the light source, the lower the delay difference at which one can still observe interference – i.e. the more precisely the depth of the scattering structure can be located. This interference signal value corresponds to exactly one layer of the sample. By shifting the reference mirror, layers of different depths can be ‘focused’ and measured with OCT. By moving the measuring beam transversely to the z-direction, one can create a three-dimensional image.
In the case of the FD-OCT, the interference is evaluated spectrally, that is to say for each wavelength. This is done by a spectrometer (SD-OCT) or the use of light from a spectrally tuned laser light source (SS-OCT). The FD-OCT makes the process significantly faster, since all back reflections of the sample are measured simultaneously.
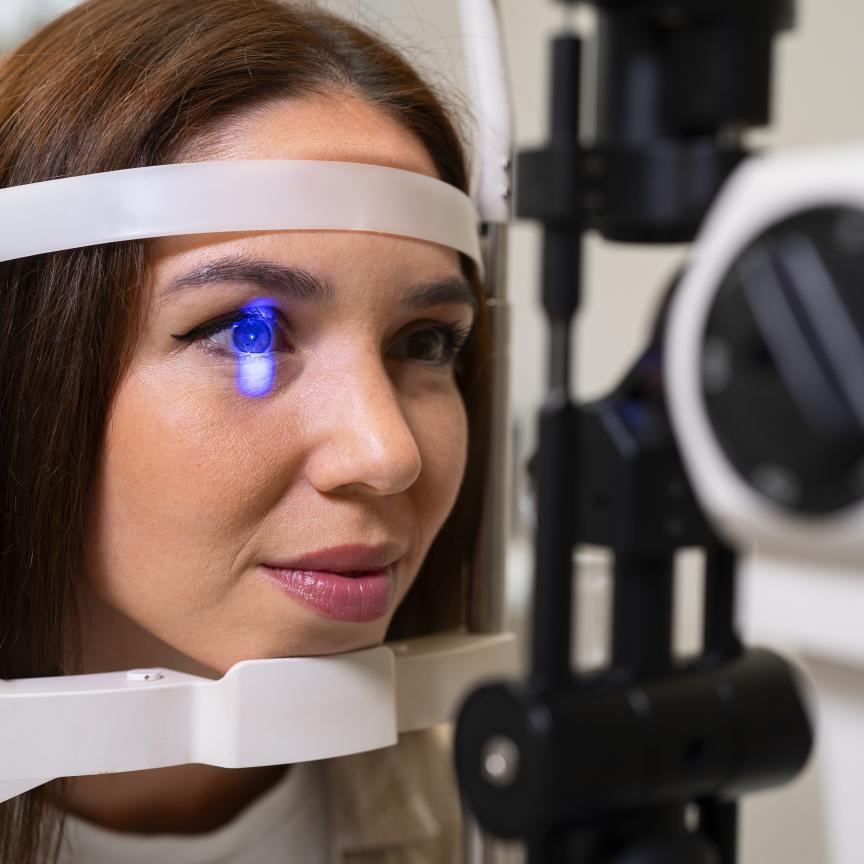
The first application of OCT in clinics is in opthalmology. However, OCT in research is found in almost all medical areas.
OCT swallable diagnostic capsules
One application of OCT in clinical development is inside swallowable microscopy capsules. Leading here is the research group led by Dr Guillermo Tearney, of Massachusetts General Hospital and Harvard Medical School. The prototypes implement SS-OCT. A micromotor inside the capsule rotates the light beam at about 40 revolutions per second, imaging the esophagus and the upper intestinal tract in spiral zones. The 11 x 25mm capsule hangs on a glass fibre, which connects it to the OCT system. ‘A diagnostic run takes about five minutes and provides images with an axial resolution of 10µm and a lateral resolution of about 30µm,’ explained Tearney.
This method eliminates the sampling errors associated with endoscopic biopsy, according to Tearney. ‘After the measurement, the capsule is withdrawn, disinfected and reused. The process is simple, fast and cost-effective. We hope to get the device commercialised in one to two years,’ he said, adding the biggest challenge is decreasing the cost of the technology, so it can be used to screen millions of people for pre-cancerous lesions and early cancers at the point of care.

Image taken by the swallable OCT diagnostic capsule. Credit: Tearney Lab
The use of FD-OCT in surgical microscopes or systems is another application in development. The most important feature of these systems is speed, said Dr Marc Krug, co-founder of OptoMedical Technologies, a spin-off of the Medical Laser Center of the University of Lübeck. ‘Intraoperative OCTs need to deliver images in real time, because the surgeon is at work on or in humans.‘
The company has developed a system for use in ophthalmic surgical procedures with a frame rate of 27 images per second (B scan). In opthalmology, an A-scan corresponds to a single depth profile of the eye. A B-scan is a combination of sequential A-scans, achieved by scanning the OCT beam laterally, and is more useful to surgeons.
‘[With OptoMedical’s system] currently, intra-operative applications provide 35,000 A-scans per second, which are processed in real-time at 27 frames per second, which the surgeon perceives as a fluid representation,’ noted Krug.
Since most monitoring devices can display a maximum of 60 images per second, one is already close to the maximum useful value. Faster systems that extend into the megahertz range for the A-scan rates are then more interesting for real-time 3D applications.
OCT at home
Usually, OCT devices are bulky, expensive and require training to be able to use them. Portable and low-cost systems are in development designed for home use by patients, who could monitor their eyes in between appointments with their optician. This could improve the treatment of macular degeneration (AMD), a disease that primarily affects older people. ‘Compared with clinical systems, such a device would have to be reduced in size and price by an order of magnitude and the handling should be simplified,’ said Dr Peter Koch, scientist at the Medical Laser Center Lübeck (MLL).
Scientists at the MLL have developed a form of OCT, known as OA-FF-TD-OCT (off-axis full-field TD-OCT), which makes it easier to design small and inexpensive OCT devices.
Normally, OCT scans the tissue point by point. With the new method, the whole object is illuminated and the reflected light is displayed on a camera. In this way, one can replace expensive galvanometer scanners with cheap cameras. The image quality is lower than the previous OCTs, but it is enough to see if something has changed in the eye.
‘The first measurements show that the technology also works with patients with previous illnesses and that even elderly patients can use the device independently,’ noted Peter Koch.
Measurements taken by the patients would be evaluated by software, and on suspcion of a change in the ocular fundus, the patient would be referred to their ophthalmologist.
Wasatch Photonics has also developed a patient-usable device for Notal Vision in this area. The device generates OCT images, which are analysed by an algorithm based on artificial intelligence (AI) of the Notal Vision Diagnostic Clinic (NVDC). The physician has access to the daily tests and logs in, if necessary.
The Notal Home OCT, which is based on the SD-OCT system, started a clinical trial in the United States in October. The first targeted application is to monitor exudative (wet) age-related macular degeneration (eAMD) patients at home between regularly scheduled clinic assessments.
Increasing the speed of OCT
Professor Robert Huber from the Institute for Biomedical Optics, University of Lübeck, developed an extremely fast light source to increase the speed of OCT: the fourier domain mode locking (FDML) laser. This technique provides a continuous frequency swept light wave stored in a long optical fibre spool in the laser cavity, the whole rainbow at once, so to speak.
Today, FDML lasers are used for light sources in SS-OCT devices. Such systems are often termed megahertz-OCT (MHz-OCT). SS-OCT is regarded as the next generation of FD-OCT and is now slowly entering in the market, but is typically more expensive than SD-OCT. In SS-OCT, one tunes the wavelengths of the light source rapidly and detects time-resolved the interference signal.
In cardiology, MHz-OCT using FDML technology has enabled a technique called Heartbeat-OCT, which enables high-quality OCT datasets of an entire coronary artery in vivo in less than a heartbeat – which dramatically reduces motion artifacts.
In surgical interventions there can be a big advantage of FDML-based MHz-OCT because of its capability to perform 4D imaging, i.e. full three-dimensional imaging and reconstruction at video volume repetition rates (25 volumes per second or more).
For this application, Optores recently launched the 4D live MHz-OCT using FDML lasers.
The system combines SS-OCT, full-field OCT and numerical reconstruction. This method provides phase stable volumetric images. The advantage is that the additional components needed for conventional OCT can be compensated numerically with a computer, making lensless imaging by reconstruction of the scattered waves possible.
Another new OCT method, holoscopy, which combines OCT with digital holography to rapidly capture structures three-dimensionally. In this case, a displacement of the object plane in any plane of the sample interior is numerically simulated, meaning that mechanical movement for focusing is replaced by numerical calculation.
In contrast to scanning systems, backscattered light from all depths is detected with the same efficiency. Outside the focus, the captured images are focused numerically by wavefront propagation. Therefore, even at high resolutions, no additional scanning across the depth is necessary. One can create high-resolution volumetric imaging in a fraction of the usual measurement time.
From the phase of the OCT measurement data, it is possible to show the smallest changes in the refractive index or movements in the nanometre range. In this way, one can visualise the movement of retinal blood vessels and the surrounding tissue when the pulse wave triggered by the heartbeat impinges. Although the effects are so small, they can be measured with high accuracy.
With this holoscopy method, three-dimensional structures can be displayed even faster. Christian Lührs, head of the OCT technology team at Thorlabs, developed the first compact demonstrator as part of his diploma thesis. Together with Thorlabs, he developed a device prototype, which works without imaging optics. Resolution and sensitivity in this 3D optical imaging process are independent of focusing.
A high-speed area camera records wavelength-dependent holograms of the sample. By means of reference light, the electric wave fields of the light scattered in the sample are calculated in the camera plane and restored in an axially displaced reconstruction plane.
‘The reconstructed wave field in this plane, which may lie inside the sample, is mathematically superimposed again with a reference light beam in order to obtain a two-dimensional numerical interferogram that can be evaluated in the manner of the OCT,’ explained Lührs . The pixel-by-pixel OCT evaluation of the interferogram then provides depth information from a layer of the sample in the vicinity of the reconstruction plane.’
Together, the researchers have increased the measuring speed to such an extent that, in the future, internal tissue structures can be viewed three-dimensionally, as in a 3D video without any imaging optics. ‘In contrast to previous methods, which had to approach all points of the tissue to be examined in succession, one can capture a complete sample volume simultaneously with this method,’ he added.
In holoscopy all information is there and one can focus on all the desired places, only that this focusing does not require a mirror, but only computational time, which is getting cheaper.
Thorlab uses MEMS-VCSEL light sources, which make it possible to scan from a few kilohertz to a few megahertz. The high coherence length of this arrangement allows image depths of up to a few metres. In addition, disturbing image artifacts can be eliminated.
But even much smaller changes are detectable. For example, the team of Professor Gereon Hüttmann, from the Institute for Biomedical Optics, and Thorlabs, was able – for the first time – to visualise processes in the photoreceptors of the retina of a living human using FF-SS-OCT. This isn’t possible with traditional OCT, because it cannot differentiate between the nanometre-wide changes in the outer segments of the eye from naturally occurring micron-sized movements. In addition, aberrations of the eye prevent the representation of individual visual cells. Numerical aberration correction can compensate the aberrations of the eye in the subsequent images, so that individual photoreceptors become resolvable. Combined with a phase-sensitive evaluation of complex OCT data, this makes it possible to directly visualise the reaction of individual photoreceptors to incident light.
Analysing data
The fact that modern OCT measurements on the eye generate data volumes of around 500MB is no surprise, given the high resolution and the fast imaging speed of the procedures. And with the other applications it looks similar. These data also need to be analysed.
NinePoint Medical develops OCT systems that are being used for gastroenterology research. Using their SS-OCT system, scientists can examine the esophagus and other areas for tissue abnormalities that may not be visible using conventional medical imaging techniques. The technology has an axial resolution of 7µm in tissue, can image 3mm deep and produce 6cm long volumetric scans of luminal organs.

Ninepoint's NvisionVLE system allows clinicians to evaluate the esophageal tissue microstructure and mark areas of suspicion. Credit: Ninepoint
NinePoint Medical recently received FDA clearance for a software product that uses AI algorithms to analyse the OCT image data in real-time and colourise the three most commonly used esophageal VLE image features to aid image review. According to the company, it is the first AI product of its kind commercially available for OCT imaging in any medical field.
In the August 2018 issue of Nature Medicine, researchers report on the success of using deep learning (Jeffrey De Fauw, et.al.). The computer system was able to make diagnoses within seconds with an accuracy of 94 per cent.