Cardiovascular disease (CVD) is the leading cause of death in Europe1 and is estimated to cost the EU economy almost €196 billion a year2. Governments across the continent are striving to improve the quality of care for CVD patients while at the same time reducing the cost burden.
With point of care (POC) testing, diagnostic tests can be carried out at the patient’s side rather than in a laboratory, which not only helps to improve patient outcomes, but also improves hospital resource management and supports more preventative and patient-centric approaches in healthcare.
Two independent EU projects have been working on the development of POC devices for different types of CVD – one for detecting heart attacks in emergency rooms, and one for diagnosing CVD before symptoms present – and they show potential for changing the way CVD sufferers are diagnosed, treated and managed.
Patients presenting at the hospital with chest pain require immediate diagnosis and treatment in order to save as much cardiac tissue as possible.
For detecting evidence of cardiac damage, doctors will measure the level of the cardiac troponin I (cTnI) biomarker – a protein excreted by the heart following a heart attack – in the blood in order to decide on the best possible treatment.
Today, the standard procedure for blood tests is to send a sample to a clinical laboratory. Results normally take at least one hour to reach the physician; such a long turnaround time can delay treatment and lead to sub-optimal patient management.
Since 2014, an EU-funded project has been investigating the analytical and clinical feasibility of detecting the cTnI biomarker at the point of care, which could not only reduce the time it takes to diagnose and treat suspected heart attacks, but help to reduce overcrowding in emergency departments and allow hospital resources to be managed more efficiently.
The ‘Lab 2 Go’ project involved six hospitals across Europe (Austria, France, Germany, the Netherlands, and the United Kingdom) and was supported by a number of European-based companies working together to develop and prepare the cTnI test for commercialisation.
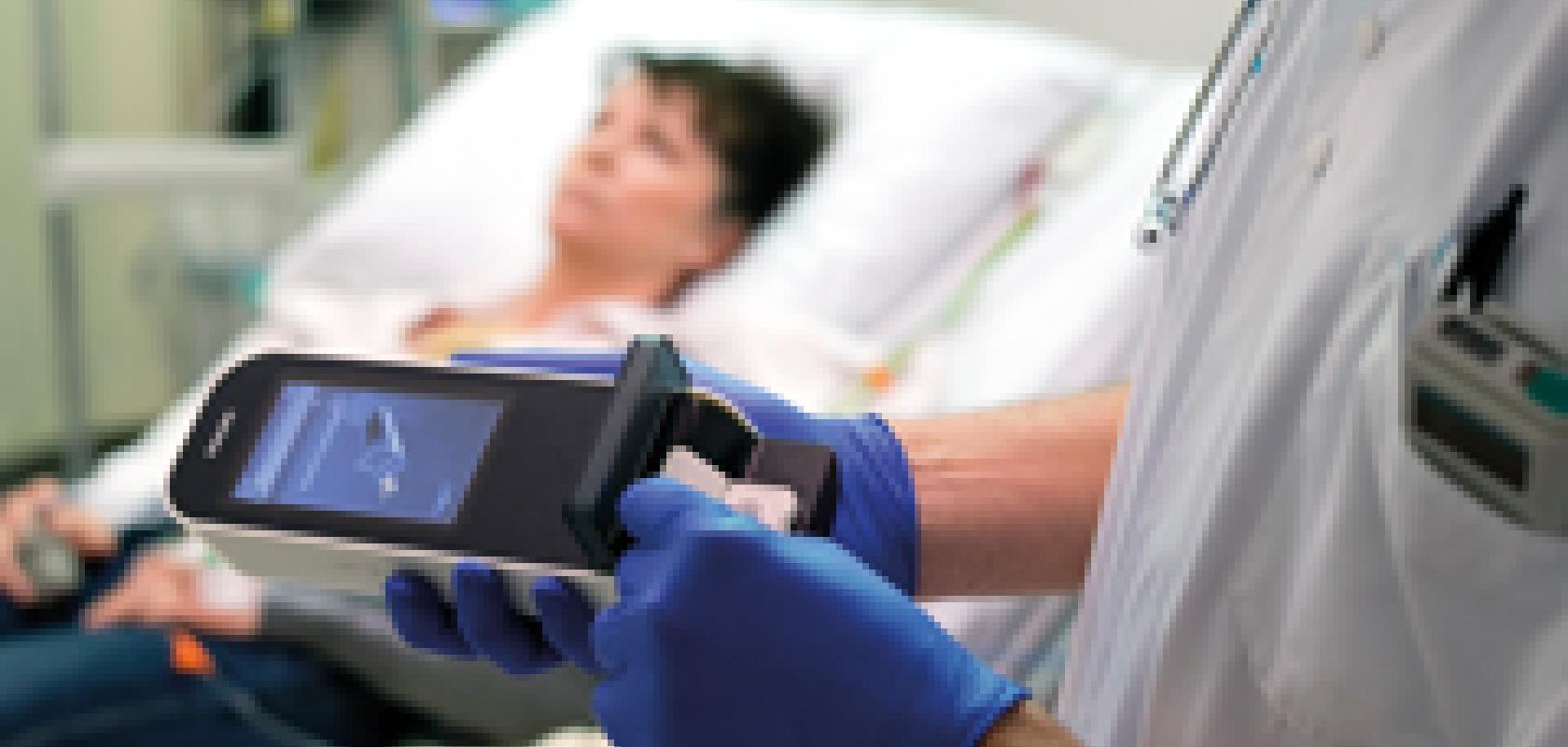
In May, the project resulted in the commercial launch of a handheld POC device, the Philips Minicare I-20 system, in several countries in Europe – including the UK, Germany, the Netherlands and Belgium. The Minicare I-20 was tested in real-life acute care settings within the Lab 2 Go project, demonstrating the system’s potential for measuring cTnI values accurately, near the patient in the emergency department, with a turnaround time of less than 10 minutes.
‘If you normally send a sample to a lab, a lot of actions need to be taken, from an administrative and logistics point of view, but also in the lab – in general, it would take an hour, but it can be longer outside of normal working hours, to get a result back,’ said Michel Simons, marketing director of Handheld Diagnostics at Philips. ‘Using the Minicare system, with a single drop of blood taken from the patient’s finger, we can generate a result at the patient’s bedside within 10 minutes. The physician can immediately take the result into consideration to make an informed decision on what the next step should be for treatment. So, you are speeding up the whole treatment process by testing… by the bedside.’
The Minicare system consists of a handheld analyser, software, and a single-use disposable cartridge containing the application-specific test.
Once the cartridge is inserted into the Minicare analyser, the patient’s blood is taken via a finger prick. The blood is filtered and fills the inner reaction chamber containing the individual immunoassay reagents.
Using Philips’ Magnotech biosensor technology – which is based on the controlled movement of magnetic nanoparticles – specific molecules relating to target antibodies in the particles are captured. An electromagnet draws the particles to the surface, where those particles that have captured a target molecule are then bound. A reverse magnetic field quickly separates the bound and unbound particles and, through optical detection, the amount of bound and unbound particles is quantified, which allows the analyte concentration in the sample to be determined. The test results are then displayed on the handheld analyser.
In addition to improving the treatment time of the patient, these types of POC handheld instruments can be operated by staff in the emergency room rather than in the lab, improving the use of hospital resources. ‘Laboratory tests that previously had to be carried out by highly trained lab technicians can now be carried out by any healthcare professional, such as a nurse, quickly and easily at the patient’s bedside,’ noted Simons. ‘This is possible because there are several fail-safes in the design of the technology, which ensure that a mistake cannot be made, and it will alarm the operator if something is not normal.’
This opens the door for the use of POC devices at the primary care level and in remote settings with no laboratory infrastructure.
A shift in healthcare
Although the Minicare system is currently only being deployed in emergency departments, the device could be applied to a wider variety of healthcare settings with the expanding application of POC molecular diagnostics. ‘We have started the introduction of the Minicare system in emergency rooms, particularly because the applications we are currently developing – for example identifying cardiac biomarkers – are typically of use in the emergency setting,’ Simons remarked. ‘We are also looking into the medical services setting – such as in an ambulance or a GP office.’
Testing patients closer to the point of care will become more important as the healthcare field shifts towards the early detection of disease. Until recently, care has been focused on disease treatment rather than prevention; in general, patients are not tested until clinical symptoms develop and they visit the doctor. As a consequence, many diseases are not diagnosed until it is too late for effective treatment.
The use of POC testing supports more patient-centred and preventative approaches to healthcare delivery, which is the key to both better health and lower healthcare costs.
‘If you look to healthcare in general, you see that there is a change in the whole healthcare environment,’ Simons explained. ‘Traditionally, there was a lot of investment going into building large hospitals and healthcare facilities, and now we see that the care is being taken out of the hospitals and being brought closer to where the patient is. Taking care out of the hospital and closer to the patient means that medical decisions can be made faster, but it is also less expensive to diagnose and monitor patients,’ he said.
The success of a potential shift from curative to preventive medicine relies, in part, on the development of portable diagnostic and monitoring devices for point-of-care testing.
‘With the change in healthcare, I think that POC in general as a market will continue to grow – if you look at the current growth figures within in vitro diagnostics (IVD), then you see that POC IVD is growing more strongly than the more traditional IVD market. I think that is a good indication of the future for POC,’ Simons continued. ‘I think you will see more and more systems that are designed for use closer to the patient in the healthcare field.’
Early detection
Another consortium of researchers and technology companies has been developing a mobile, low-cost POC screening device for the early detection of cardiovascular disease. Launched in 2015, the Horizon 2020 ‘Cardis’ project aims to demonstrate a laser Doppler vibrometer for screening arterial stiffness, and detecting stenosis (a narrowing or constriction of the inner surface of the artery) and heart failure in a clinical setting.
Identifying individuals at risk for CVD allows for early intervention – such as lifestyle changes or medication – which plays an important part in halting, or even reversing, the pathological processes associated with CVD.
Assessing arterial stiffness – an early marker for hypertension – through the measurement of aortic pulse wave velocity (aPWV) is included in the latest guidelines for CVD risk prediction produced by the European Society of Hypertension and European Society of Cardiology.
However, today, there are no tools available for screening a large population for these parameters under primary care, and individuals that are considered to be at a low or moderate risk are often undiagnosed.
Over the past few years, research groups, including the University of Ghent and the Queen Mary University of London, have demonstrated that mechanical vibrations induced by cardiovascular dynamics can propagate up to the skin, where they can be detected using laser vibrometry.
A laser Doppler vibrometer (LDV) is an instrument used to make non-contact vibration measurements of a surface. For assessing arterial stiffness, the laser beam from the LDV is directed onto the surface of the skin overlying the artery under investigation, and the vibration amplitude and frequency are extracted from the Doppler shift of the reflected laser beam frequency caused by the motion of the surface. The output of an LDV is generally a continuous analogue voltage that is directly proportional to the target velocity component along the direction of the laser beam.
In addition to measuring aortic and local PWV, an LDV device can also be used to detect vibrations induced by disturbed blood flow in stenosed arteries, along with cardiac contraction abnormalities via measurements on the chest. By measuring these different indicators, an LDV can lead to an improved screening and assessment of cardiovascular risk at the point of care.
The project will run through to 2018, and will be managed by Imec, through Imec’s associated laboratory located at Ghent University. The Medtronic Bakken Research Centre in the Netherlands will be responsible for the scientific and technical coordination of the project.
Other industrial, academic and clinical partners of the Cardis project include: SIOS Messtechnik (Germany); University College Cork Tyndall (Ireland); Institut National de la Recherche Médicale; Queen Mary University of London (United Kingdom); Universiteit Maastricht (Netherlands); Ghent University; and Fundico (Belgium).
References
Shrinking the optics for Point of care
Point of care (POC) instruments by their nature have to be small, low cost and give fast results while still maintaining accuracy. Shrinking the optics without compromising the optical output is one of the challenges of designing POC devices.
‘Optics and detection technologies must keep pace with advances in micro-fluidics, test automation, and detection reaction chemistries,’ commented Colleen Dwyer, marketing and communications coordinator at spectral sensing manufacturer Pixelteq.
Oliver Pust, director of sales and marketing at Delta Optical Thin Film, added that the optics have to be able to cope with the adverse effects of large angles of incidence (AOI) typically found in compact instruments.
The smaller optical setup means that wider angles of incidence are necessary to collect enough light. Greater AOI can lead to polarisation effects, along with the centre or edge wavelengths shifting towards the blue end of the spectrum. Delta Optical Thin Film develops high-performance filters for POC applications designed to minimise these effects. The company also manufactures the filters in volume, thereby keeping costs down, another requirement for POC instruments.
Pixelteq has developed its PixelSensor multispectral sensor in order to reduce the size and complexity of optical components and detectors for quantitative polymerase chain reaction (qPCR) POC instruments.
Real-time or quantitative polymerase chain reaction is a DNA amplification technique and a major enabling technology for molecular diagnostic testing at the patient’s bedside. The technique eliminates the need for post-amplification electrophoresis and combines amplification and detection within the same instrument.
PixelSensor packages up to eight channels in a compact 9 x 9mm leadless chip carrier (LCC) package. Optical filters are coated directly onto photodiodes, and the number of channels and wavelengths can be customised for a specific diagnostic test chemistry.
Jennifer Odom, senior OEM account manager and business development, biomedical market, PixelTeq noted that while the company is focusing PixelSensor on PCR applications initially, it could be used more broadly as a detector in other POC instruments based on optical detection, such as fluorescence, chemiluminescence or absorbance. ‘If, for example, heart attacks are being diagnosed at the bedside by labelling blood markers with fluorescence or chemiluminescence tags, then PixelSensor’s filters could be customised to the relevant wavelengths for those markers,’ she concluded.