The Code of Hammurabi, a well-preserved Babylonian law code and one of world’s oldest deciphered writings, specified medical malpractice regulations in the early days of eye surgery. In 1754 B.C., the ophthalmologist’s penalties varied with the economic status of the patient:
‘If a doctor operates ... on the eye of a patrician who loses his eye in consequence, his hands shall be cut off. In the case of a slave, if the surgeon has caused his death the penalty was to replace him by another, and if he made the slave lose his eye, he shall pay half his value’[1].
Modern laser technologies are advancing eye treatment and diagnoses in ways that the Babylonians could not have imagined, thus helping patients to keep their eyes and surgeons their hands.
From ancient times to modern, advances in instrumentation have led to more refined techniques for treating a wider range of ophthalmological problems.
And with the global ophthalmic laser market valued at $854 million in 2016, and forecast to reach $1.5 billion by 2025[2], the ophthalmic sector represents a significant opportunity for laser manufacturers, optics companies and medical system suppliers.
Precision LASIK
In 1980, IBM Research laboratory scientist Rangaswamy Srinivasan discovered that an excimer laser could etch living tissue without thermal damage to the surrounding area. Shortly after, Steven Trokel of Columbia University published the results of efforts to reshape the eye’s cornea using a laser. The cornea is the transparent layer at the front of the eye, which helps the eye to focus.
Now, corrective laser eye surgery has become the go-to procedure for correcting vision impairments including myopia, hyperopia, and astigmatism. The most common version is laser-assisted in situ keratomileusis, or LASIK, in which the ophthalmologist reshapes the eye’s cornea to achieve the same visual acuity that would have been achieved with glasses or contacts.
Here, the surgeon creates a thin flap in the cornea, originally using a mechanical tool with an oscillating blade called a microkeratome. But with the introduction of femtosecond lasers in 2001, cutting this flap became a more reliable procedure.
‘The original lasers worked at 5kHz; now they’re as fast as 500kHz,’ said Edward Manche, director of cornea and refractive surgery at the Stanford Eye Laser Centre. Originally, cutting a flap with a laser could take over two minutes; while a mechanical tool took 10 to 12 seconds. Now, cutting a flap with a laser takes ‘only 12 to 18 seconds’.
More importantly, femtosecond lasers reduce biomechanical complications because they’re less likely to cut an irregular or uneven flap, as was often the case with a mechanical kerotome, which weakened the cornea.
Additionally, wavefront technology that was developed alongside femtosecond lasers uses a non-contact laser to shine a grid pattern into the eye. The beam passes through all of the eye’s structures and reflects off the retina at the back of the eye. When the beam returns, it’s imaged with a CCD and can then be analysed to calculate a pattern for the cornea that would neutralise all aberrations.
‘That’s another significant advance, because you can get a precise measurement of the eye and attempt to control for that during the laser ablation,’ Manche added.

The iDesign system uses wavefront acquisition and Fourier algorithms to help create a personalised treatment plan
Manche and his team use Abbott Medical Optics’ (AMO) iDesign System for vision correction procedures. The system captures five different wavefront measurements in a single sequence, measuring up to 1,257 micro-refractions over a 7mm diameter pupil. That’s a fivefold improvement in resolution compared to previous wavefront acquisition systems. ‘The same technology was used to measure the curvature of the James Webb Space Telescope mirror,’ noted Manche. ‘We’re able to use that on actual eyes.’
The wavefront image drives the final step, AMO’s Star S4 IR excimer laser, as it ablates the corneal surface to the desired pattern.
SMILE for minimally invasive surgery
In late 2016, the United States Food and Drug Administration (FDA) approved small incision lenticule extraction (SMILE) surgery. Here, a femtosecond laser creates a disc-shaped incision, or lenticule, within the cornea. The lenticule is then removed through an access incision of less than 6mm, all in a single treatment step with a single laser. Removing the precalculated lenticule changes the shape of the cornea and achieves the desired correction.
‘The concept is that you only need the femtosecond laser, you don’t need an excimer laser to reshape the cornea,’ said Manche. It also avoids cutting the flap, which is one of the weak points of LASIK surgery. Instead, the outer cornea remains intact, thus avoiding biomechanical weakening. Further, the SMILE incision cuts below the sub-basal nerves at the periphery of the cornea, thus reducing the number of severed corneal nerves that are unavoidable in LASIK.
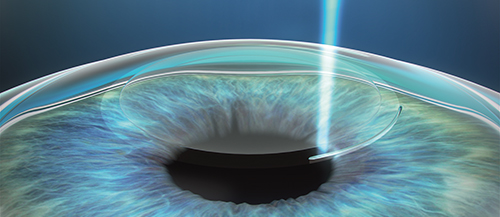
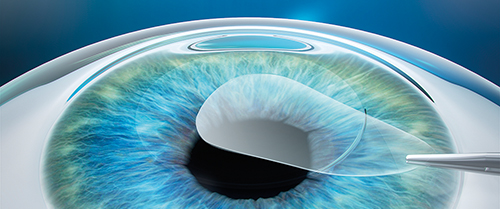
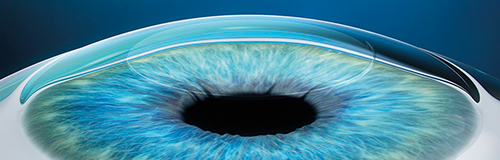
SMILE procedure with VisuMax laser: A refractive lenticule and small incision are created inside the cornea; the lenticule is removed through the small incision; and the new cornea shape has the desired refractive correction
Carl Zeiss Meditec’s VisuMax femtosecond laser is the only system currently approved for SMILE (Zeiss Relex Smile) in the United States. VisuMax’s curved laser interface fits apex-to-apex with the cornea, avoiding any misshaping. Further, it avoids the need for an excimer laser, which relies on fixed laser energy that can interact differently with the cornea based on the hydration properties of the tissue; if the corneal tissue is left exposed for too long it can dehydrate, resulting in over-treatment with the laser. Similarly, if the tissue is overly hydrated, the laser energy is essentially soaked up by the excess fluid and has less impact altering the tissue.
‘You can do very precise 3D cutting within the cornea to obtain the correct shape,' said Paul Rousseau, senior director of refractives for Zeiss North America. ‘We target a specific shape and achieve that without the decreased laser beam fluence that can impact the quality of an excimer based LASIK treatment.’
SMILE’s minimally invasive, flapless procedure brings refractive surgery to patients who might be sensitive to the biomechanical instabilities introduced by LASIK. ‘The lenticule extraction opens a wider part of the market,’ noted Rousseau.
But the two procedures are – and will – continue to be complementary, with each offering advantages for certain conditions. ‘LASIK has been around for more than 30 years, with numerous refinements. It will be around for a long time, with more advances to come,’ added Stanford’s Manche.
Taking the heat off with MicroPulse
Ophthalmology laser therapies extend beyond refractive surgery, with advances helping to treat other eye diseases, such as glaucoma, where extra fluid in the front part of the eye damages the optic nerve; and diabetic macular edema, which can cause swelling of the retina, or the light-sensitive tissue at the back of the eye, leading to blindness. Both can be helped with laser treatments that have traditionally involved a conventional, continuous-wave laser. In conventional laser treatments, a rapid temperature rise in the target tissue creates blanching and a high thermal spread, which can cause tissue damage and scarring that spreads overtime.
To solve this problem, laser-based medical system manufacturer, Iridex, developed its MicroPulse laser therapy, which allows the tissue to cool between laser pulses. This minimises tissue damage, reducing or eliminating risks, and can be used alone or as an adjunct to other treatments.

Iridex’s new Cyclo G6 glaucoma laser system
‘With MicroPulse, the continuous-wave laser is chopped into repetitive on and off pulses,’ explained Joan Stauffer, director of clinical marketing and education at Iridex. ‘MicroPulse allows tissue to cool between laser pulses and reduces the thermal build-up.’ The doctor selects a duty cycle that determines the pulse rate.
Iridex first developed MicroPulse using an 810nm laser, which at the time was a new wavelength for this use, according to Stauffer. This led to incorporating MicroPulse into 577nm and 532nm, which are more accepted by physicians. ‘Our IQ 577 and IQ 532 laser systems offer two treatment modes: MicroPulse and continuous-wave, so physicians can use one laser in many ways to treat both retinal disorders and glaucoma,’ she said.
In an eye with glaucoma, either the eye is producing too much fluid, or the fluid does not drain properly – causing a build-up of intraocular pressure (IOP). This high IOP contributes to optic nerve damage, which results in vision loss starting at the periphery. One common glaucoma treatment is laser trabeculoplasty, intended to reduce excess pressure in the eye by increasing fluid outflow through the trabecular meshwork. With MicroPulse, the trabecular tissue meshwork remains intact without signs of tissue damage.
MicroPulse technology has also been incorporated into Iridex’s new Cyclo G6 glaucoma laser system for MicroPulse transscleral cyclophotocoagulation (TSCPC), a repeatable and minimally invasive approach to slow the progression of glaucoma. ‘I have been impressed that the MicroPulse P3 therapy with the Cyclo G6 can deliver pressure lowering in a broad range of glaucomas, including mild and moderate patients who I want to free from the adverse effects of drops,’ said Dr Rolando Toyos from Toyos Clinic in Nashville, Tennessee.
References
[1] Fishman, R S. (1999). The History of Ophthalmology. Bull Hist Med, 73:174-175.
[2] Business Wire (2017). www.businesswire.com/news/home/20170626005690/en/Global-Ophthalmic-Lase…

OCT image of cornea pre-Descemet’s layer
Optical tools play an important role in modelling ophthalmologic diseases, too, and in some cases lead to interesting discoveries. In 2016, investigators used Wasatch Photonics’ optical coherence tomography (OCT) spectrometer to image a new layer inside the cornea, the frontmost part of the eye.
OCT is an optical imaging technique known for its high-sensitivity and speed, now a mainstay in ophthalmic diagnostics. It uses interference patterns in the spectral domain to provide information about scattering at different depths, thus providing images at sub-micron-level resolution. Recent developments of broad-bandwidth light sources and high speed, large pixel-number cameras have led to ultrahigh-resolution OCT technology.
During a study to image the cellular structure of healthy and diseased human corneas, researchers at the University of Waterloo, led by Kostdinka Bizheva, used the Wasatch OCT spectrometer to ‘confirm the presence of a previously unknown layer inside the eye,’ said Nishant Mohan, vice president of Wasatch’s OCT business unit. The existence of this layer had been controversial in ophthalmology for a long time: while surgeons had seen it during operations and first reported it in 2013, they had not imaged it in vivo.
The 15µm-thick ‘pre-Descemet’s layer’ plays an important role in corneal surgery, as precise techniques require knowledge of the exact locations of the corneal sublayer boundaries. When inserting an air bubble between corneal layers during a standard graft procedure, it turns out that the new layer is the one to target, rather than the Descemet’s membrane itself that separates the cornea body from underlying tissue. The pre-Descemet’s layer can be easily separated from the rest of the cornea, and indeed separates the main cornea from its bottom membrane.
Detection of this layer is expected to be of significance both in corneal surgery and pathology. ‘It is ongoing work now to see how this layer changes in diseased patients,’ said Mohan. Ultrahigh resolution OCT imaging will help to image these changes and offer better understanding and treatments.
Reference
Bizheva, Kostadinka, et al. In Vivo Imaging and Morphometry of the Human Pre-Descemet’s Layer and Endothelium With Ultrahigh-Resolution Optical Coherence Tomography In Vivo UHR-OCT of Posterior Corneal Layers. Investigative ophthalmology & visual science. 57.6 (2016): 2782-2787


